How To Determine Lone Pairs In A Molecule
Lone pairs hydrogens carbon counterions organic implies confusion causes Geometry chemistry vsepr theory lone pairs molecules electron shapes molecule bonding geometries chem vsper predicting atom molekul bentuk libretexts tetrahedral Molecular structure and polarity (4.6) – chemistry 110
Number of Lone Pairs and Bonding Pairs for H2O (Water) - YouTube
Vsepr atoms theory bonded electron molecules least adopt depending upon Lone pairs atom chemistry number calculate central organic Lone molecules atom central pairs which has chemistry pair
Molecules in which the central atom has no lone pairs
How to determine the number of lone pairsMolecular dipole How to determine hybridization: a shortcut – master organic chemistryHybridization sp lone atoms determine pairs examples pair chemistry attached organic alkyne nitrile shortcut sum.
Lone pairs number determine chemistry formulaIn the lewis dot structure for nh_3, the central atom of the molecule Valence shell electron pair repulsion theoryLone pairs bond angles.

How to determine the number of lone pairs
Molecular polarity electron vsepr geometries lone lattices predicted maximize cosmicJimchem: vsepr theory How to calculate the number of lone pairs on a central atom, organicMolecular structure and polarity.
9.7: the shapes of moleculesLone pairs & bond angles Molecules in which the central atom has lone pairs10.3: vsper theory- the effect of lone pairs.

Dipole molecular molecule polarity dichloromethane dipoles determine lone pairs
Structure geometry molecular chemistry theory pairs bonds chem electron shape pair polarity density geometries vsepr angle regions vsper lone aroundLone h2o bonding Geometry molecular lone pairs vsepr model geometries bonding shapes chemistry models theory molecules vsper covalent effect electron basic bent chemStructure pairs molecular lone electron chemistry geometry pair table polarity geometries molecules vsepr between angle bonding bonds trigonal examples linear.
Organic chemistry blind spots: hidden hydrogens, lone pairs, andMolecular structure and polarity · chemistry Pairs determine bonding nitrogen chemistryAtom central lone molecules has which pairs geometry pair.
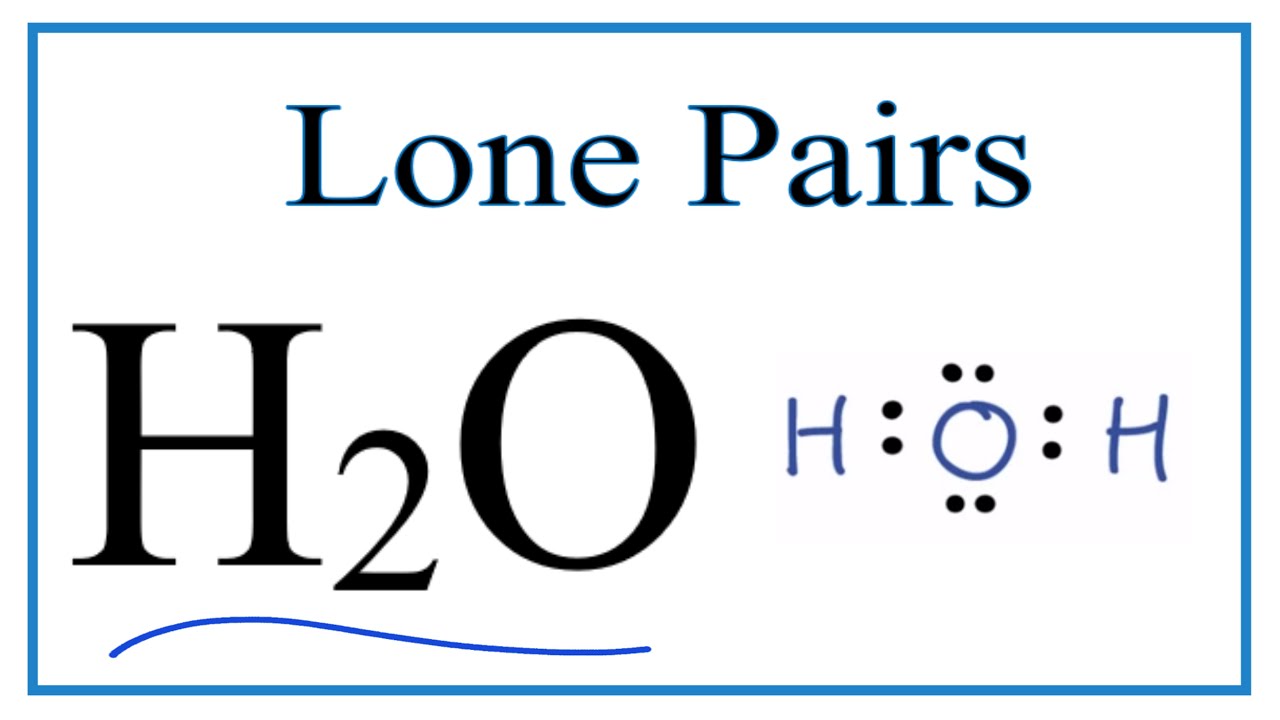
Electron pair shapes theory vsepr molecules valence shell repulsion chemistry molecular bond linear shape geometry chart lone bonding geometries chem
Geometry molecular chart electron molecule vsepr chemistry atom lone xef2 predict atoms bonded steric electronsNumber of lone pairs and bonding pairs for h2o (water) .
.


Molecular Structure and Polarity · Chemistry

Molecules in Which the Central Atom has Lone Pairs - The Way of Chemistry

9.7: The Shapes of Molecules - Chemistry LibreTexts

How To Determine Hybridization: A Shortcut – Master Organic Chemistry

How to calculate the number of lone pairs on a central atom, organic

Molecular Structure and Polarity | Chemistry
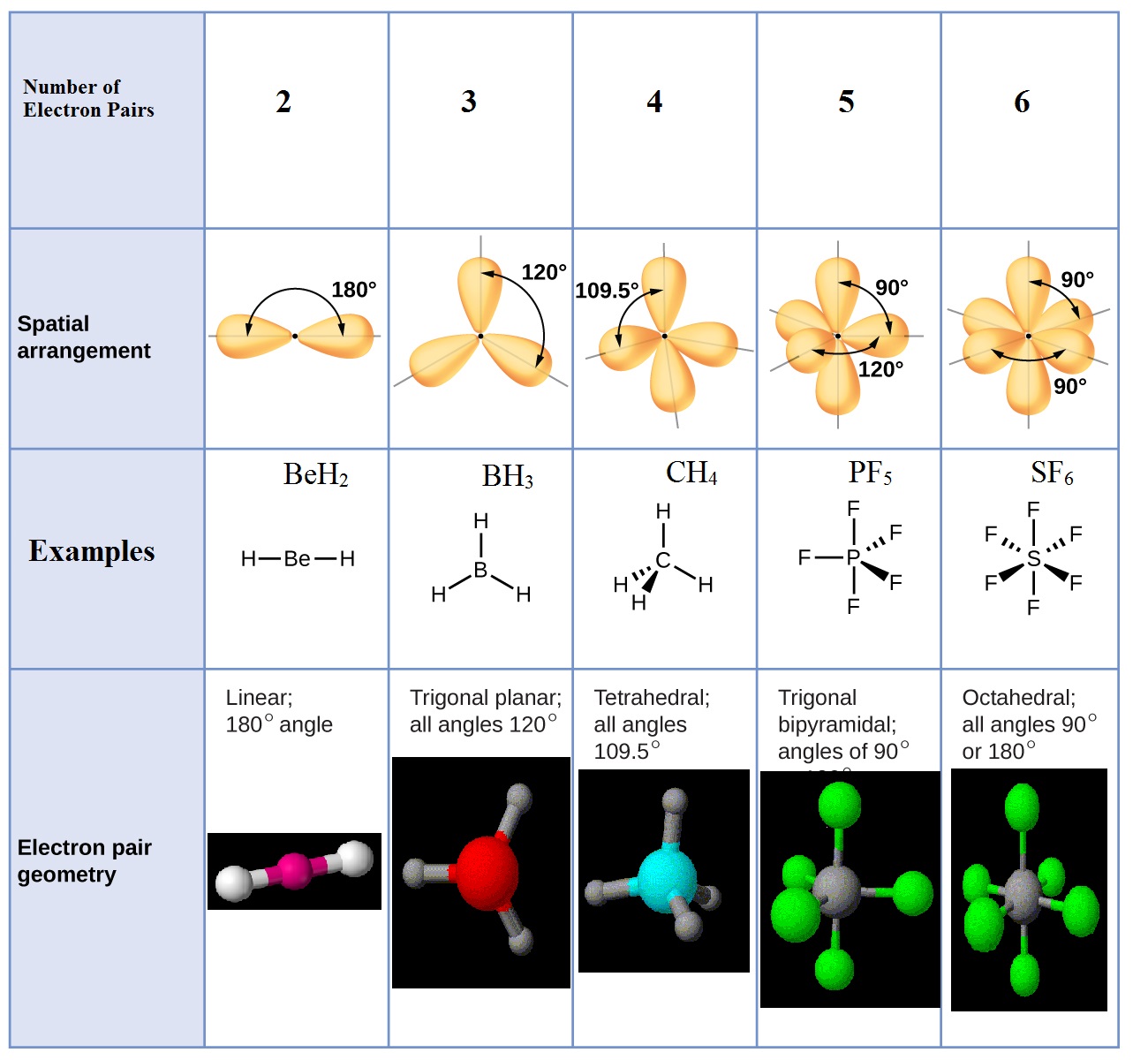
Jimchem: VSEPR Theory

Molecules in Which the Central Atom has no Lone Pairs - The Way of
