Atoms Emit Energy As Light When
Photons atoms photon electrons energy light atom wave being called electron when absorption emit absorb release waves state radiation absorbs Physical science 7.3h Light atom does any exist energy photon wavelength levels difference emit nucleus form
Atomic Hydrogen Absorption Spectrum
Light atoms spectroscopy ppt powerpoint presentation energy Proposed method to cause an atom to emit the same light as another atom Aurora (northern lights)
Light absorb emit atoms science
Atom emission energy light atomsEmission atom atoms photon particle vsc apollo classes lights charged chapter2 lsc excess Spectroscopy absorption hydrogen spectrum interactionAtomic hydrogen absorption spectrum.
Emit electrons atomRadiation electromagnetic Solved consider heated lithium atoms that emit photons ofEnergy photon lines emission atom absorption levels gif atomic example emitting emit emitted state down equal exactly.

Which system below will emit a light of higher energy
Atoms emit bohr matterCan light exist in an atom? Hydrogen spectra emission bohr lines visible wavelengths atom spectral atoms specific chemistry emit absorb11.3 emission of energy by atom.
Emission and absorption linesLight emit electron atoms ppt emitted photon Light excited atoms photon aurora atom oxygen emit green look part bullmer shells do spectrum colour would energies two brightDefinition of light energy.

Scientific explorer: atoms part 2: atoms and light
Atom emit proposedAtoms, electrons and photons What is an emission spectrum? use the bohr model to explain why theHow do atoms emit light?.
Lecture notes for chapter 11Emit atoms lithium heated photons problem Electron excited electrons light flame does notes atoms state atom ground describe photon energy photons emit work tests lecture back.


Atomic Hydrogen Absorption Spectrum

What is an emission spectrum? Use the Bohr model to explain why the

11.3 Emission of Energy by Atom - ChemistrySAANguyen

Scientific Explorer: Atoms Part 2: Atoms and Light

Lecture Notes for Chapter 11

Solved Consider heated lithium atoms that emit photons of | Chegg.com

Emission and Absorption Lines
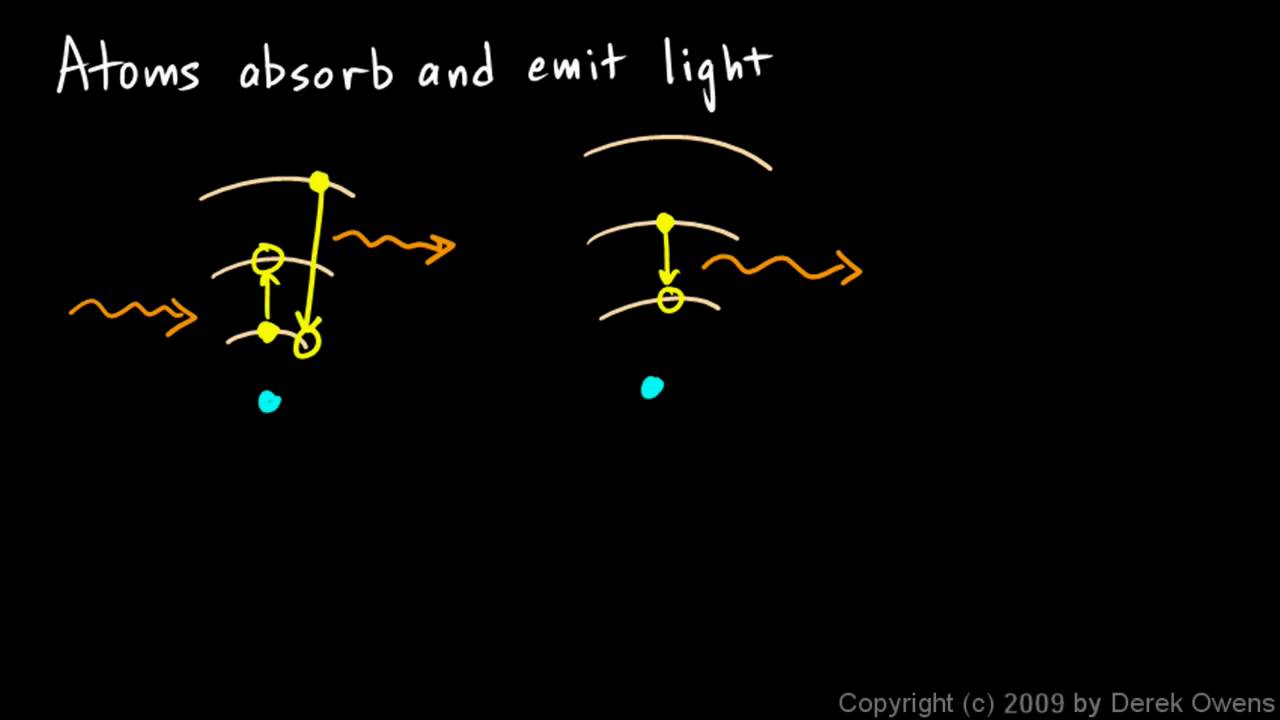
Physical Science 7.3h - Atoms Absorb and Emit Light - YouTube

Definition of Light Energy
